RESEARCH
GENERAL INFORMATION ON CLINICAL TRIALS
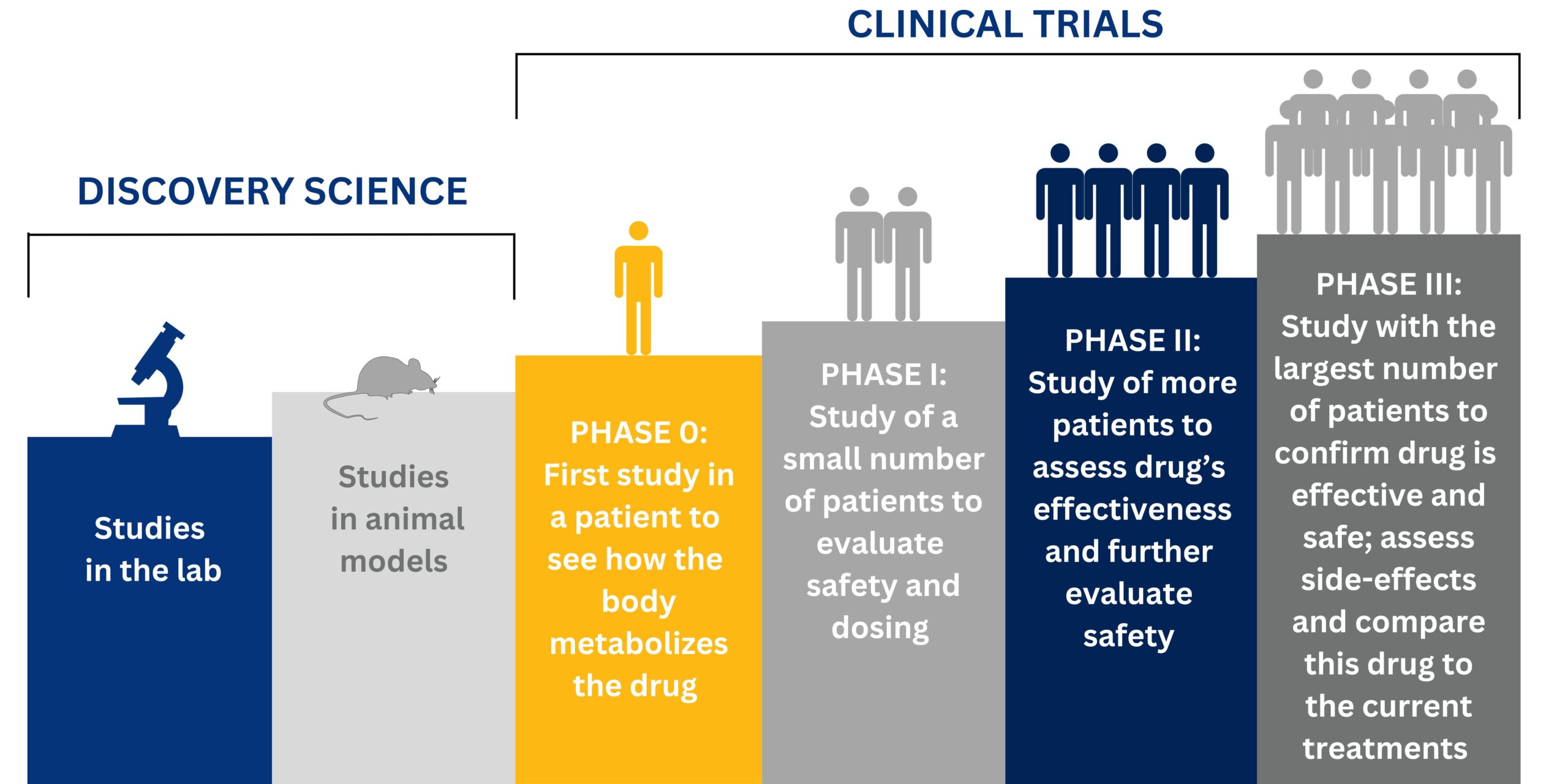
What is a Clinical Trial?
A clinical trial is research to test new medications for different diagnoses and to see how the medication works in different people. For a new medication to be approved by the FDA and used by physicians it first needs to go through several phases of clinical trials and prove that it is beneficial. Clinical trials is necessary in order to provide patients with safer and more effective options of treatment.
How Does a Clinical Trial Work?
Clinical trials require pharmaceutical companies to come up with a research plan specific to the questions they are trying to answer. Sites that participate in the clinical trial have investigators who are usually doctors to help answer these questions by finding participants who are eligible and different treatments are used and then compared. The different treatments may include the study drug, approved drugs, and placebo (no active ingredients).
What kinds of questions do clinical trials ask?
Researchers are focused on potential side effects, whether it improves the diagnosis, if it works differently in certain individuals, and if it is better than the drugs that have already been approved by the FDA. These questions are general and could have additional questions within each general category.
Pros and Cons of Becoming a Participant in a Clinical Trial:
PROS
-
You are closely monitored throughout the duration of the trial receiving additional care apart from your regular doctor that you see for your illness.
-
Medical care, the visits you attend, and procedures used are at no cost to you.
-
A possibility of receiving treatment that is not available to the public.
CONS
-
Time commitment for the visits and procedures that are done may be more than care you receive from your regular doctors
-
There is no guarantee that your condition improves
-
Side effects that are known and unknown could potentially occur